Historical Landmarks in Our Understanding of Proteins
•
•
•
•
•
•
•
•
•
•
•
•
1838 The name "protein" (from the Greek proteios, "primary") was suggested by Berzelius for
the complex organic nitrogen-rich substance found in the cells of all animals and plants.
1819-1904
Most of the 20 common amino acids found in proteins were discovered.
1864 Hoppe-Seyler crystallized, and named, the protein hemoglobin.
1894 Fischer proposed a lock-and-key analogy for enzyme-substrate interactions.
1897 Buchner and Buchner showed that cell-free extracts of yeast can ferment sucrose to form
carbon dioxide and ethanol, thereby laying the foundations of enzymology.
1926 Svedberg developed the first analytical ultracentrifuge and used it to estimate the correct
molecular weight of hemoglobin.
1933 Tiselius introduced electrophoresis for separating proteins in solution.
1942 Martin and Synge developed chromatography, a technique now widely used to separate
proteins.
1951 Pauling and Corey proposed the structure of a helical conformation of a chain of L-amino
acids -- the alpha helix -- and the structure of the beta sheet, both of which were later found
in many proteins.
1955 Sanger completed the analysis of the amino acid sequence of insulin, the first protein to have
its amino acid sequence determined.
1956 Ingram produced the first protein fingerprints, showing that the difference between sicklecell hemoglobin and normal hemoglobin is due to a change in a single amino acid.
1963 Monod, Jacob, and Changeux recognized that many enzymes are regulated through
allosteric changes in their conformation.
Number & Size Distribution of Cellular Proteins
Size & Shape Comparisons of Proteins
Protein Structure and Function
• Protein Structure
– Primary structure - amino acid sequence.
– Secondary structure - formation of a helices and b sheets.
– Tertiary structure - the three-dimensional conformation of a
polypeptide chain.
– Quaternary structure - formation of a protein molecule as a
complex of more than one polypeptide
chain.
Protein Structure and Function
• Protein Function
– Enzymes - proteases, synthetases, polymerases, kinases
– Structural - extracellular
collagen, elastin
intracellular
tubulin, actin, a-keratin
– Transport - serum albumin, hemoglobin, transferrin
– Motor - myosin, kinesin, dynein
– Storage - ferritin, ovalbumin, calmodulin
– Signaling - insulin, nerve growth factor, integrins
– Receptor - acetylcholine receptor, insulin receptor, EGF
receptor
– Gene regulatory - lactose repressor, homeodomain proteins
– Special purpose - green fluorescent protein, glue proteins
Protein Structure and Function
• Protein Structure
– Primary structure - amino acid sequence.
– Secondary structure - formation of a helices and b sheets.
– Tertiary structure - the three-dimensional conformation of a
polypeptide chain.
– Quaternary structure - formation of a protein molecule as a
complex of more than one polypeptide
chain.
Amino Acids
Codon Usage Table
Protein Folding
Protein Denaturation & Refolding
Protein confirmation is determined solely by its amino acid sequence
Protein Structure and Function
• Protein Structure
– Primary structure - amino acid sequence.
– Secondary structure - formation of a helices and b sheets.
– Tertiary structure - the three-dimensional conformation of a
polypeptide chain.
– Quaternary structure - formation of a protein molecule as a
complex of more than one polypeptide
chain.
a helix Secondary Structure
b sheet Secondary Structure
Noncovalent Bonds
Noncovalent Bonds
Hydrogen Bonds in Proteins
Noncovalent Bonds
Noncovalent Bonds
Noncovalent Bonds
Noncovalent Bonds
b sheet Secondary Structure
Antiparallel b sheet
Parallel b sheet
a helix Interactions with Phospholipids
Protein Structure and Function
• Protein Structure
– Primary structure - amino acid sequence.
– Secondary structure - formation of a helices and b sheets.
– Tertiary structure - the three-dimensional conformation of a
polypeptide chain.
– Quaternary structure - formation of a protein molecule as a
complex of more than one polypeptide
chain.
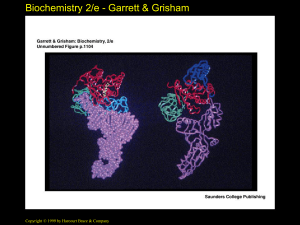
Tertiary Structure
Tertiary Structure
Cytochrome b
Lactate dehydrogenase
IgG light chain
Structural Importance in Protein Function
Coiled-coiled Structure of Multiple a helices
• A
single a helix with amino acids
a and d being nonpolar.
• B
two a helices wrap around each
other with one nonpolar side
chain interacting with the
nonpolar side chain of the
other. The hydrophilic side
chains are exposed to the
aqueous environment.
• C
atomic structure of a coiled-coil
showing the nonpolar
interactions in red
Protein Structure and Function
• Protein Structure
– Primary structure - amino acid sequence.
– Secondary structure - formation of a helices and b sheets.
– Tertiary structure - the three-dimensional conformation of a
polypeptide chain.
– Quaternary structure - formation of a protein molecule as a
complex of more than one polypeptide
chain.
Quaternary Structure
Quaternary Structure
Hemoglobin
Protein - Protein Interactions
• A protein with just one
binding site can form a
dimer with an identical
protein.
• Identical proteins with two
different binding sites can
form a long helical filament.
• If the two binding sites are
located appropriately to each
other, the protein subunits
can form a closed ring
instead of a helix.
Collagen and Elastin
Disulfide bonds






![First observation of Hb D-Ouled Rabah [beta19(B1)Asn>Lys] in the](http://s1.studylibtr.com/store/data/003346881_1-fc6465a17750760535fb52bbef4ddf81-300x300.png)